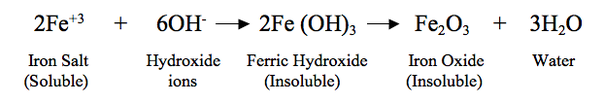For high crop yields adequate plant nutrition is very important. It is relatively easy to accomplish the needed fertility levels for the major and secondary nutrients. However, for the micronutrients, this can be a little more difficult. Although plants need only minute amounts of these trace elements, they play very significant roles in the growth and production of the plants. Because of unfavorable soil conditions such as pH, organic matter content and aeration, the availability of micronutrients to the roots is reduced. Application of trace elements to the soil to increase its fertility level is not as easy as the applications for the major or secondary nutrients. Increasingly, chelates have been used to overcome the unfavorable soil conditions and supply micronutrients to plants.
The micronutrient cations iron, zinc, copper and manganese are relatively unavailable in most soil solutions when provided as inorganic salts. This insolubility is more pronounced when the pH of the soil is higher than 5, because the metals can react to form insoluble metal oxides. One such example is the reaction of the ferric ions with hydroxyl ions where the resulting precipitate a reddish-brown oxide (rust):
This reaction reduces the quantity of iron which is available to the plants because both Fe(OH)3 (ferric hydroxide) and Fe2O3 (Iron oxide) are unavailable to the plants. Thus in this example, even though the iron is chemically present in the soil, it is of no value to the plants.
Because of undesirable reactions such as these, metal chelates have become an important means of supplying the micronutrients to the plants. In the above example, if instead of the inorganic salt of iron, an iron chelate had been used, the ferric iron would have been protected from precipitation, and the iron available for plant use increased.
Chelates are now commonly used to prevent or correct micronutrient deficiencies as well as deficiencies of other micronutrients, particularly in high pH, calcareous soils, and to a lesser extent in acid soils.
Successful use of chelates has been reported world-wide. At the present, chelates are not only used in calcareous soils to correct nutrient deficiencies, but they are also used in low pH soils to supply micronutrients. Both soil and foliar applications are widely used, however the trend has been switching from soil application to foliar application because of the higher effectiveness and faster response obtained in the plants from the chelates through this method. In addition to the soil and foliar applications, chelates have also been used in trunk injections to fruit tress and in seed coatings with high degrees of success.
Chelates are widely accepted as being better than the inorganic salts in correcting certain micronutrient deficiencies in plants under a variety of conditions. The advantages of using chelates on crop production are:
1) The chelating agents will protect the cations chelated by them from fixation or leaching caused by unfavorable soil conditions, such as high soil pH. The chelates are more stable than the inorganic salts in the soil. In high pH soils, the inorganic salts may become unavailable to plants because of the reactions between the cations and hydroxyl (OH) groups or other compounds in the soils. The cations from the inorganic salts may also be subject to leaching and not be able to be reached by plant roots. Application of chelates as substitutes for inorganic salts will overcome these problems and become more effective than the inorganic salts.
2) Some of the chelating agents have the inherent properties of being able to promote increased uptake of the chelated minerals. Lignosulphonates and amino acids, or hydrolized protein, have been reported to fall into this category. Because of these unique properties, minerals applied as certain chelates have better uptake by plants.
3) Certain types of chelated minerals may be translocated throughout the plant better than the inorganic salts. Iron amino acid chelates applied to one leaf in corn plants were better distributed to the other parts of plants than FeSO4. More ions are translocated within the plants when they are applied as chelates than as inorganic salts. Chelating agents may help the mineral translocation within plants.
4) Some of the chelates, such as lignosulphonate and amino acid chelates, have none or low, phytotoxicity. This none, or low, phytotoxicity property enables the user to apply the chelates to the seeds or to the foliage of plants without danger of injury. In organic salts and some synthetic chelated minerals, because of their high salt index, frequently damage plant leaves or inhibit seed germination. More caution must be taken to avoid the adverse effect of inorganic salts to plants.
5) Chelates will not only provide nutrients to plants, but may also reduce the level of excess nutrients in the plants. The excess nutrients may become inactive by binding with the chelating agents and thus increase the degree of internal nutrient balance. A more balanced nutrient status is one major key to higher crop yield.
6) Some of the chelating agents have hormone-like effects on plants. Application of these chelates may stimulate the plant growth even though there are no visual nutrient deficiencies. This can be due, partly, to the more balanced nutrient status.
Because of undesirable reactions such as these, metal chelates have become an important means of supplying the micronutrients to the plants. In the above example, if instead of the inorganic salt of iron, an iron chelate had been used, the ferric iron would have been protected from precipitation, and the iron available for plant use increased.
Chelates are now commonly used to prevent or correct micronutrient deficiencies as well as deficiencies of other micronutrients, particularly in high pH, calcareous soils, and to a lesser extent in acid soils.
Successful use of chelates has been reported world-wide. At the present, chelates are not only used in calcareous soils to correct nutrient deficiencies, but they are also used in low pH soils to supply micronutrients. Both soil and foliar applications are widely used, however the trend has been switching from soil application to foliar application because of the higher effectiveness and faster response obtained in the plants from the chelates through this method. In addition to the soil and foliar applications, chelates have also been used in trunk injections to fruit tress and in seed coatings with high degrees of success.
Chelates are widely accepted as being better than the inorganic salts in correcting certain micronutrient deficiencies in plants under a variety of conditions. The advantages of using chelates on crop production are:
1) The chelating agents will protect the cations chelated by them from fixation or leaching caused by unfavorable soil conditions, such as high soil pH. The chelates are more stable than the inorganic salts in the soil. In high pH soils, the inorganic salts may become unavailable to plants because of the reactions between the cations and hydroxyl (OH) groups or other compounds in the soils. The cations from the inorganic salts may also be subject to leaching and not be able to be reached by plant roots. Application of chelates as substitutes for inorganic salts will overcome these problems and become more effective than the inorganic salts.
2) Some of the chelating agents have the inherent properties of being able to promote increased uptake of the chelated minerals. Lignosulphonates and amino acids, or hydrolized protein, have been reported to fall into this category. Because of these unique properties, minerals applied as certain chelates have better uptake by plants.
3) Certain types of chelated minerals may be translocated throughout the plant better than the inorganic salts. Iron amino acid chelates applied to one leaf in corn plants were better distributed to the other parts of plants than FeSO4. More ions are translocated within the plants when they are applied as chelates than as inorganic salts. Chelating agents may help the mineral translocation within plants.
4) Some of the chelates, such as lignosulphonate and amino acid chelates, have none or low, phytotoxicity. This none, or low, phytotoxicity property enables the user to apply the chelates to the seeds or to the foliage of plants without danger of injury. In organic salts and some synthetic chelated minerals, because of their high salt index, frequently damage plant leaves or inhibit seed germination. More caution must be taken to avoid the adverse effect of inorganic salts to plants.
5) Chelates will not only provide nutrients to plants, but may also reduce the level of excess nutrients in the plants. The excess nutrients may become inactive by binding with the chelating agents and thus increase the degree of internal nutrient balance. A more balanced nutrient status is one major key to higher crop yield.
6) Some of the chelating agents have hormone-like effects on plants. Application of these chelates may stimulate the plant growth even though there are no visual nutrient deficiencies. This can be due, partly, to the more balanced nutrient status.