The term Chelate is a chemical name derived from the Greek word “chele” – meaning claw. The function of a chelate is to protect trace elements from becoming unavailable to plants.
A Chelate is an organic compound which can chemically complex with a positively-charged metal cation such as zinc, manganese, iron, copper, magnesium or calcium. The chelate (organic molecule) surrounds the positively-charged metal protecting it from becoming unavailable to plants. Therefore chelated trace elements are more available to plants.
By remaining in solution at high pH values chelates ensure that the protected trace elements are not subject to precipitation as insoluble oxides or hydroxides. The chelated form of a trace element does not react with phosphate in the soil, nor is it affected by the higher pH that follows liming. Although chelated trace elements are protected against soil reaction, this form of micro-nutrient is readily assimilated by growing plants. Thus organic chelates offer an opportunity to supply necessary manganese, copper, zinc, cobalt, molybdenum and other metallic elements in a manner that lime, phosphates or soil pH cannot adversely influence their availability to plants.
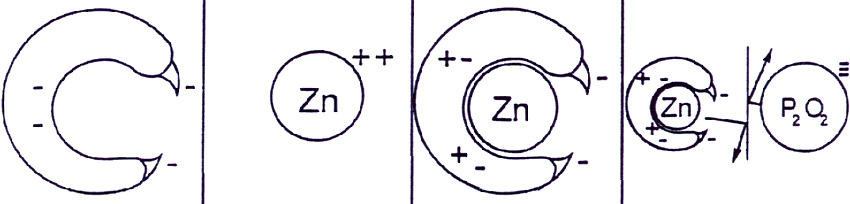
Figure 1 depicts the chemistry of zinc chelation and demonstrates that in this protected form zinc is unavailable to react with phosphate. Thus zinc phosphate does not precipitate, in which zinc would be unavailable to the plant. Therefore chelation maximizes availability of zinc to the plant.
Figure 1: Schematic representation of the zinc chelation process.
By remaining in solution at high pH values chelates ensure that the protected trace elements are not subject to precipitation as insoluble oxides or hydroxides. The chelated form of a trace element does not react with phosphate in the soil, nor is it affected by the higher pH that follows liming. Although chelated trace elements are protected against soil reaction, this form of micro-nutrient is readily assimilated by growing plants. Thus organic chelates offer an opportunity to supply necessary manganese, copper, zinc, cobalt, molybdenum and other metallic elements in a manner that lime, phosphates or soil pH cannot adversely influence their availability to plants.
Figure 1 depicts the chemistry of zinc chelation and demonstrates that in this protected form zinc is unavailable to react with phosphate. Thus zinc phosphate does not precipitate, in which zinc would be unavailable to the plant. Therefore chelation maximizes availability of zinc to the plant.
Figure 1: Schematic representation of the zinc chelation process.
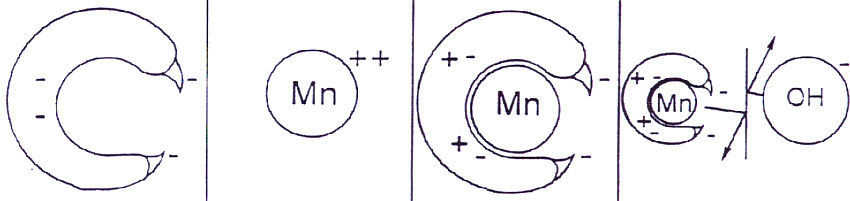
Figure 2 depicts the chemistry of Manganese chelation and shows that in this protected form manganese is not affected by high pH soil. Thus Manganese Hydroxide is not precipitated, which is insoluble and would result in manganese being unavailable to the plant. Therefore chelation maximizes availability of manganese to the plant.
Figure 2: Schematic representation of the Manganese chelation process.
Figure 2: Schematic representation of the Manganese chelation process.