An important property of the metal chelates is their stability constant. This number is specific for each metal and chelate molecule and defines the relative stability of the metal chelate in terms of the ratio of bound metal in the chelate to free metal ion not bound in the chelate structure. This is why synthetic chelating agents form stronger bonds with some trace elements than others.
Synthetic chelating agents include:
EDTA (Ethylene diamine tetracetic acid),
DTPA (Diethylene triamine pentaecetic acid),
HEEDTA (Hydroxyethyl ethylene diamine triacetic acid),
CDTA (Cyclohexane trans 1, 2 diamino tetracetic acid) and
EDDHA (Ethylene bis Alpha-imino-2hydroxyphenyl-acetic acid).
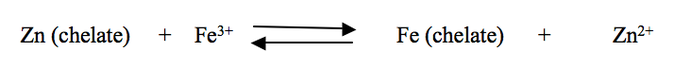
The strength of the synthetic metal chelates in decreasing order is: Iron (3) > Copper > Zinc > Iron (2) > Manganese > Calcium > Magnesium >. The most commonly used synthetic chelating agent EDTA has a strong affinity for iron, when EDTA-Zinc is used in an environment containing iron the EDTA will replace zinc with iron, becoming an iron chelate. The iron chelate is more stable than its zinc counterpart, which explains the tendency of the reaction to go to the right.
EDTA (Ethylene diamine tetracetic acid),
DTPA (Diethylene triamine pentaecetic acid),
HEEDTA (Hydroxyethyl ethylene diamine triacetic acid),
CDTA (Cyclohexane trans 1, 2 diamino tetracetic acid) and
EDDHA (Ethylene bis Alpha-imino-2hydroxyphenyl-acetic acid).
The strength of the synthetic metal chelates in decreasing order is: Iron (3) > Copper > Zinc > Iron (2) > Manganese > Calcium > Magnesium >. The most commonly used synthetic chelating agent EDTA has a strong affinity for iron, when EDTA-Zinc is used in an environment containing iron the EDTA will replace zinc with iron, becoming an iron chelate. The iron chelate is more stable than its zinc counterpart, which explains the tendency of the reaction to go to the right.
The released Zn ion is left unprotected and is subject to reaction with the soil the same as is zinc from an inorganic salt such as Zinc Sulphate (ZnSO4). It is obvious that the added metal-chelate combination must be stable within the soil if it is to have any lasting advantage.
The strength of the ligand to bind the metal ions affects the availability of the micro-nutrient to plants. Table 2 shows commonly used chelators grouped together according to their relative chelating strength. As a general rule, chelates with high stability constants (e.g. synthetic chelates) tend to hold on tightly to the mineral and do not readily release it to the plant. Chelates with lower stability constants (e.g. most organic chelates) hold onto minerals loosely. A weak chelating agent is not able to protect against hydrolysis, especially in a high pH environment. When dealing with synthetic chelates, since the metal ions must be released either within the plants or at the surface of the roots, a strong chelating agent may tightly bind the metal and not release it to the plants.
The strength of the ligand to bind the metal ions affects the availability of the micro-nutrient to plants. Table 2 shows commonly used chelators grouped together according to their relative chelating strength. As a general rule, chelates with high stability constants (e.g. synthetic chelates) tend to hold on tightly to the mineral and do not readily release it to the plant. Chelates with lower stability constants (e.g. most organic chelates) hold onto minerals loosely. A weak chelating agent is not able to protect against hydrolysis, especially in a high pH environment. When dealing with synthetic chelates, since the metal ions must be released either within the plants or at the surface of the roots, a strong chelating agent may tightly bind the metal and not release it to the plants.
Table 1. Metal chelating (complexing) agents grouped according to their chelating (complexing) strength.
| Strongest (synthetic) |
Intermediate (Long-chain natural organics) |
Weakest (Short-chain or small organics) |
|---|---|---|
| EDTA | Polyflavonoids | Citric Acids |
| HEEDTA | Ligand sulphonates* | Ascorbic Acid |
| DTPA | Humic & Fulvic Acids | Tartaric Acid |
| EDDHA | Amino Acids | Adipic Acid |
| NTA | Glutamic Acids | |
| CDT | Polyphosphates** |
* Some companies are making these synthetically.
** Polyphosphates are not organic; however, they behave similarly to organic chelate molecules.
The strongest complexing agents (synthetics) are used mainly in soil applications. Their high stability is an advantage under certain conditions, such as high soil pH (7.8 and greater). However, under other conditions, these strong chelates may lose their effectiveness. Studies have shown that EDTA was not an effective supplier of iron and manganese in high organic matter soils. Additionally, because of the high stability constant of iron-EDDHA, plant roots could not compete with EDDHA for iron. For the purpose of supplying nutrients to plants under this condition, the synthetic chelates may be considered as very poorly available.
The application rate for a synthetic chelate is very critical because they are phytotoxic to plant foliage, even when applied in the soil, unless the rate of application is accurate. When applied as foliar sprays, synthetic chelating compounds effectiveness are of modest or little benefit.
The intermediate chelating compounds are more often used in foliar application than in soil application, although some of them have been used successfully in the soil. Since these chelating agents are easily decomposed, and thus release the metal ions to plants, they are not phytotoxic to plant foliages. That makes them very suitable for foliar application. From a cost effective point of view, foliar application is less expensive than soil application in correcting micronutrient deficiencies due to the minute amounts applied. Nevertheless, they can also supply micronutrients effectively in a soil application but such a treatment requires a slightly higher rate of application.